
Invention Summary:
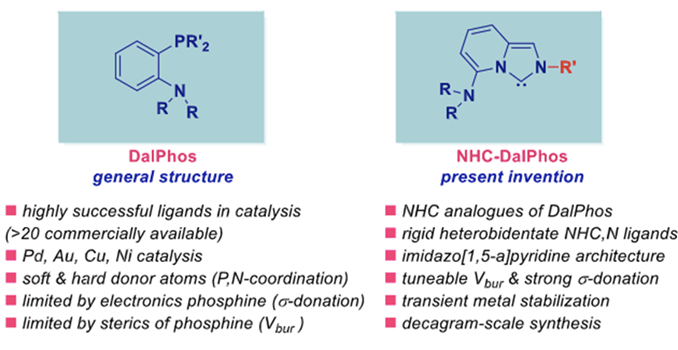
Dalphos ligands are among the most successful and widely used arlyphosphine ligands in organic catalysis. They are among the most popular ligands in the optimization and development of new processes using Au catalysis, where the stabilization of Au intermediates by five-membered chelates plays an essential role in catalysis that is not available by other families of ligands.
Rutgers’s scientists have invented amino-decorated heterobidentate L-shaped NHC ligands that are analogous to DalPhos aryl phosphine ligands. This class of ligands is focused on placing the amine substituent at the 5-position of the imidazo[1,5-a] pyridine scaffold, resulting in the rigid NHC template that enforces 1,4-relationship between the soft and hard donors, namely NHC and N, which is geometrically similar to the ortho-substitution in Dalphos ligands, where more s-donating and readily shape-modifiable carbene center replaces the phosphine. Many studies have been done to demonstrate the novel features of the ligands including their stable shape and their efficacy in producing salts and complexes that are commercially valued.
Market Applications:
• Can be used to produce salts and complexes that are widely used in many fields.
Advantages:
- Rigid NHC template that is stable and effective.
- Stronger σ-donation and better π-acceptance than phosphinesas, resulting in robust reactivity.
- Potential for facile diversification and reactivity tuning.
Intellectual Property & Development Status: Provisional patent submitted. Available for licensing and/or research collaboration.