Invention Summary:
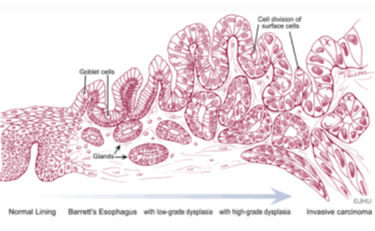
Esophageal adenocarcinoma (EAC) has a poor prognosis with a 5-year overall survival of 15-20%. In the past 3 decades, the incidence of EAC increased 600-800%. Barrett’s esophagus (BE), the precancerous lesion for EAC, has approximately 0.11 to 1.6% annual rate of progression to EAC. Currently, the guidelines for BE surveillance are periodical follow-up endoscopy, which suffers from low cost-effectiveness and inconsistent patient compliance since the incidence of EAC is low. Thus, there is an urgent need to identify biomarkers associated with a high risk of progressing to EAC.
Rutgers researchers utilizing the Illumina Methylation EPIC microarray assay for 850,000 methylation sites to study the biomarker to identify BE with high risk to develop to EAC. More than 20 hypermethylation genes are identified as promising biomarkers for screening BE patients with higher risk of progressing to esophageal adenocarcinoma.
The sensitivity, specificity and accuracy of top 20 gene hypermethylation to identify high risk patients to progress to EAC were 100%, 100% and 100% respectively, which is the best biomarkers in the market.
Advantages:
- Dramatically decreasing the cost of BE surveillance
- More accurate and sensitive test than what exists on the current market
- Early treatment before the cancer happening
- Able to estimate a patient survival rate of 100%
Market Applications:
- Identify BE patients at high risk progressing to EAC before EAC happens
- Develop a diagnostic methylation kit
Intellectual Property & Development Status: Patent pending. Available for licensing and/or research collaboration.