Invention Summary:
Hydrogen is a clean fuel that can produce electricity when combined with oxygen in a fuel cell, with water being the only by-product. Manufacturing of hydrogen through coal gasification, biomass gasification or natural gas, however, is energy-intensive and has carbon by-products. Alternatively, green hydrogen production employs electrolysis of water, which can be produced using renewable energy such as wind and solar.
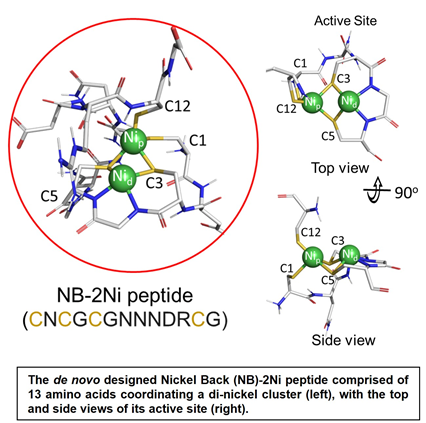
Rutgers scientists have designed a 13 L-amino acid peptide that can catalytically generate hydrogen under anaerobic conditions when complexed with two nickel ions (Ni2+) and coupled to an electron donor of sufficient reduction potential. As proof of principle, a standard photochemical assay using a photosensitive dye, results in a turnover number of 505 and a turnover frequency (TOF) of 0.2 H2/min. The peptide complex is highly stable between 20 to 90oC and pH 5.5 to 10. Depending on the coupled electron donor, it can be used to generate molecular hydrogen in a clean and sustainable way without the need for rare earth elements.
Advantages:
- Small size
- High stability at a wide range of temperatures and pH
- Redox-stable with turnover numbers > 500
- Low cost and easy to scale up
- Flexibility in engineering variant sequences
Market Applications:
- Electrocatalyst for hydrogen evolution
- Photocatalyst (coupled with photosensitizers) for hydrogen evolution
- Electrocatalyst for biofuel cells
Intellectual Property & Development Status: Patent pending. Available for licensing and/or research collaboration.